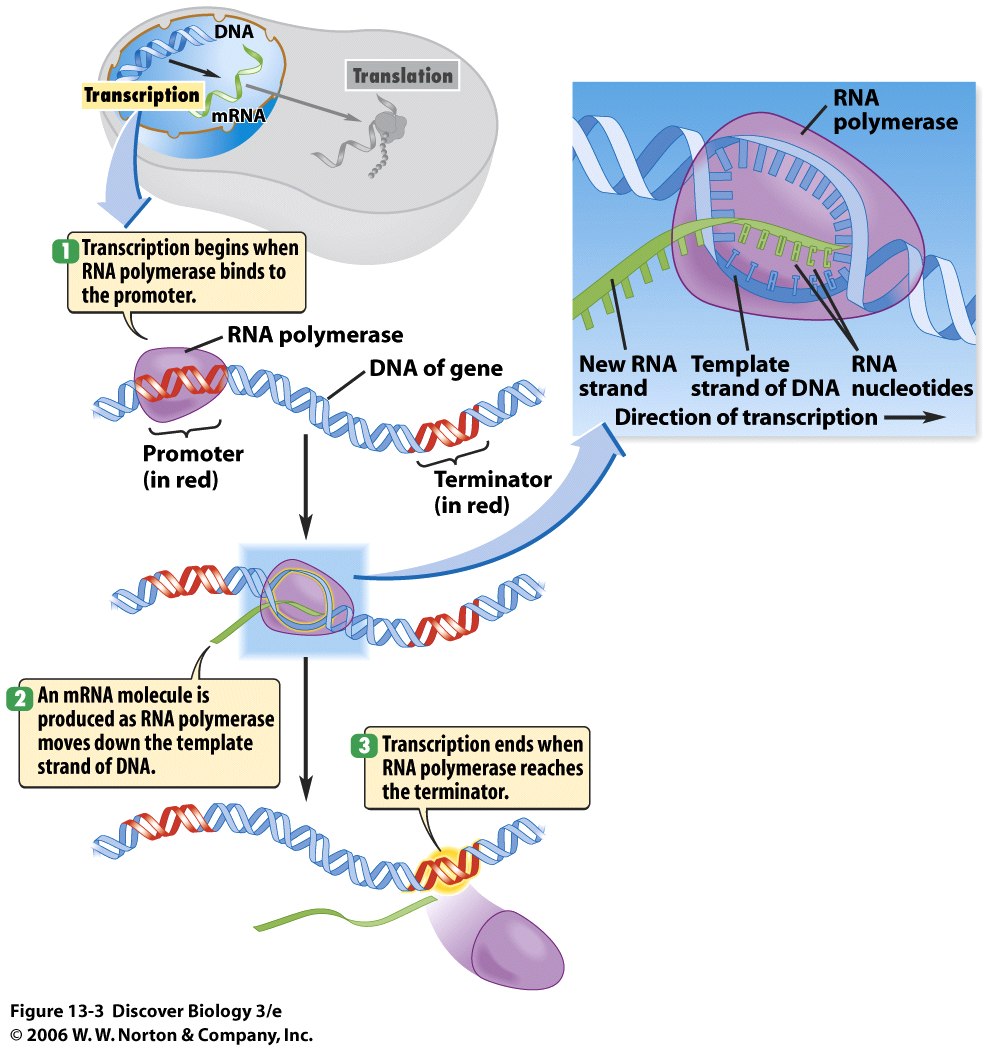
How Lettuce Can Produce Recombinant Proteins (RP's)
What is a recombinant protein you may ask? A recombinant protein is simply a protein that is made from the combination of several, different genetic material from other living\nonliving organisms. For instance, after reading Dr. Chen's article, Gene Delivery into Plant Cells for Recombinant Protein Production, I learned that the recombinant proteins he and his grad students are attempting to synthesize obtain their genetic material from mammalian, bacteria, plant, and insect cells. These proteins are responsible for the creation of the highly-valued antibodies that hopefully will help prevent viruses such as the Dengue and West Nile virus from replicating. As you can see, it is very important to study further about these proteins because they can benefit the medical world and hopefully bring an end to these disastrous epidemics. The process to producing a recombinant protein is rather simple, but really monotonous in the laboratory. Here is a diagram showing one way how recombinant proteins are made.

Today's pharmaceutical companies have approved of the use of plants such as Nicotiana benthamiana to produce the recombinant proteins ever since the development of the first plant-derived therapeutic enzyme for Gaucher's disease. Gaucher's disease is a hereditary disease that causes a buildup of fatty tissue around the liver, spleen, and bones. The reason there are a buildup of fatty issue is due to a lack of production of the key fat break-down enzyme called glucocerebrosidase. The buildup of fat around the bones actually weaken the bone tissue and can cause an increase risk for bone fractures due to too much pressure on the bones. After this new treatment, enzyme replacement therapy, was discovered; the transition to plant gene delivery was seen more beneficial than ever. Gene delivery to the transgenic Nicotiana benthamiana plants is not only cheaper in terms of manufacturing costs, but the process is really simple. All I have to do is make sure the target transgene, which is the gene that I extract from another entity and transfer it to a target host to express it, is integrated into the plant genome. Like any other living organism it is at the genome where the genes become expressed and soon produce the proteins that are encoded from those genes' DNA sequences. However, recently a new strategy has been discovered regarding an even quicker way for the recombinant proteins to be produced from the transgenic plants. It turns out that recombinant proteins can be sequenced and produced by the transgenes directly, where the transgene being inserted inside the plant cell can manually use the plant machinery to produce the RP's directly. This process is done only if the 'position effect' is eliminated. I will talk about more about the concept of the 'position effect' in my next blog, because it is a very important concept to understand in my senior project.
Lettuce is one example of a non-Nicotiana plant host that can produce recombinant proteins, however at a cost. Lettuce is known to produce higher levels of phenolics and alkaloids, which are compounds that can affect purification resins and hence add to production costs. Since there are a higher concentration of these compounds, there are a lot of FDA regulations that lettuce-produced recombinant proteins do not abide to; hence adding to more problems over manufacturing costs. For any con there has to be a pro. When lettuce undergoes the agricultural process called agroinfiltration, which I will discuss more about in my next blog, lettuce is able to produce efficient, abundant recombinant proteins. There is a study where researchers injected deconstructed viral vectors into normal, wild type lettuce hosts in order to observe the expressed pharmaceutical proteins. These researchers used a capsid protein that a combination of Norwalk virus (NVCP) and geminiviral vector genetic material to show that lettuce is efficient in producing recombinant proteins. The tested lettuce sample was able to produce the Norwalk virus like particle as efficiently as a Nicotiana plant host could do as well, where these functioning virus like particles (VLP's) can be used to induce an immune response in lab mice. As you can see they are producing a vaccine just by experimenting with commonplace lettuce that practically every individual can obtain from a farm. The production of these VLP's are very beneficial to the production of vaccines, since their similar structural characteristics to the real pathogenic viruses can aid in developing immune responses against the pathogens in lab hosts and hopefully in the future for humans as well. Imagine a world where there is a vaccine for every single kind of pathogen. Lastly, since lettuce can be easily grown and organized in controlled acreage environments this adds to another pro to using lettuce as a VLP producer, and hopefully this manageable crop can be further studied to see if it can indeed create a VLP for every virus so that vaccines can be produced.







 Here is an example of a sucker.
Here is an example of a sucker.