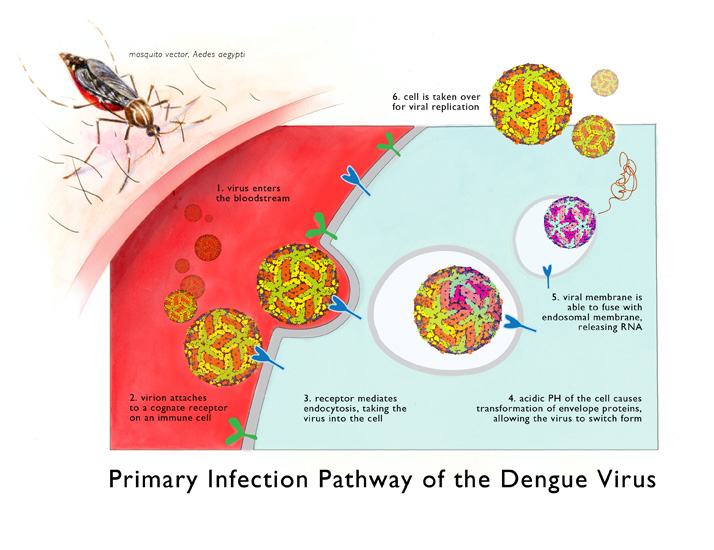
THE End Has Come

Working in the laboratory and with the graduate students at the Biodesign Institute has taught me several key tools that I will never take for granted. I have learned more about biotechnology and its relationship with microbiology than I would ever had in a common classroom setting. I would like to thank the following graduate students: Richard, Ryan Jonathan, Ashley, and Cullen for all of their help in the laboratory. They have taught how it's like to be a college student and how working in a laboratory is a great way to showcase the knowledge that I have acquired from all of my years studying at BASIS. I would also want to thank my on-site mentor, Dr.Chen and Hualing, because they trusted me working in the laboratory and having my own portable office for me to work at. These people have been great role models to me and showed how it is like to be a professional working in the laboratory in order to conduct research that has the potential of saving thousands of lives in the future. The research that I have acquired from my internship will benefit my future as I have obtained more knowledge and skills that I can apply this upcoming fall. Like I said before I have learned way better doing hands-on work than I would have learned from reading a book, and because of that I would like to say thank you to the entire Biodesign staff.
Another group that I have to thank are my counselors and my Basis mentor, Mrs. Sandor. Thank you for being there for me whenever I had questions about my research and figuring new ways to better organize the information that I have learned from the many articles that I have read over these last three months. The data tables that Mrs. Sandor has sent me are very helpful for this line of research and that I really appreciate having the chance to organize my results in a research model. Mrs. Sandor's comments and research support is something that I can never forget and I truly am thankful to have her as my mentor. So thank you again. Also, thank you Mrs. Q and Mrs. Kate for giving me this opportunity to study this line of research, because I have truly grown from this experience. I would like to thank you for keeping up with my blog posts and making sure that I have had all my assignments turned in. Again, thank you for allowing me to have a senior project in the first place, because I have truly appreciated the opportunities I have at the Biodesign Institute.
Another group that I have to thank are my counselors and my Basis mentor, Mrs. Sandor. Thank you for being there for me whenever I had questions about my research and figuring new ways to better organize the information that I have learned from the many articles that I have read over these last three months. The data tables that Mrs. Sandor has sent me are very helpful for this line of research and that I really appreciate having the chance to organize my results in a research model. Mrs. Sandor's comments and research support is something that I can never forget and I truly am thankful to have her as my mentor. So thank you again. Also, thank you Mrs. Q and Mrs. Kate for giving me this opportunity to study this line of research, because I have truly grown from this experience. I would like to thank you for keeping up with my blog posts and making sure that I have had all my assignments turned in. Again, thank you for allowing me to have a senior project in the first place, because I have truly appreciated the opportunities I have at the Biodesign Institute.


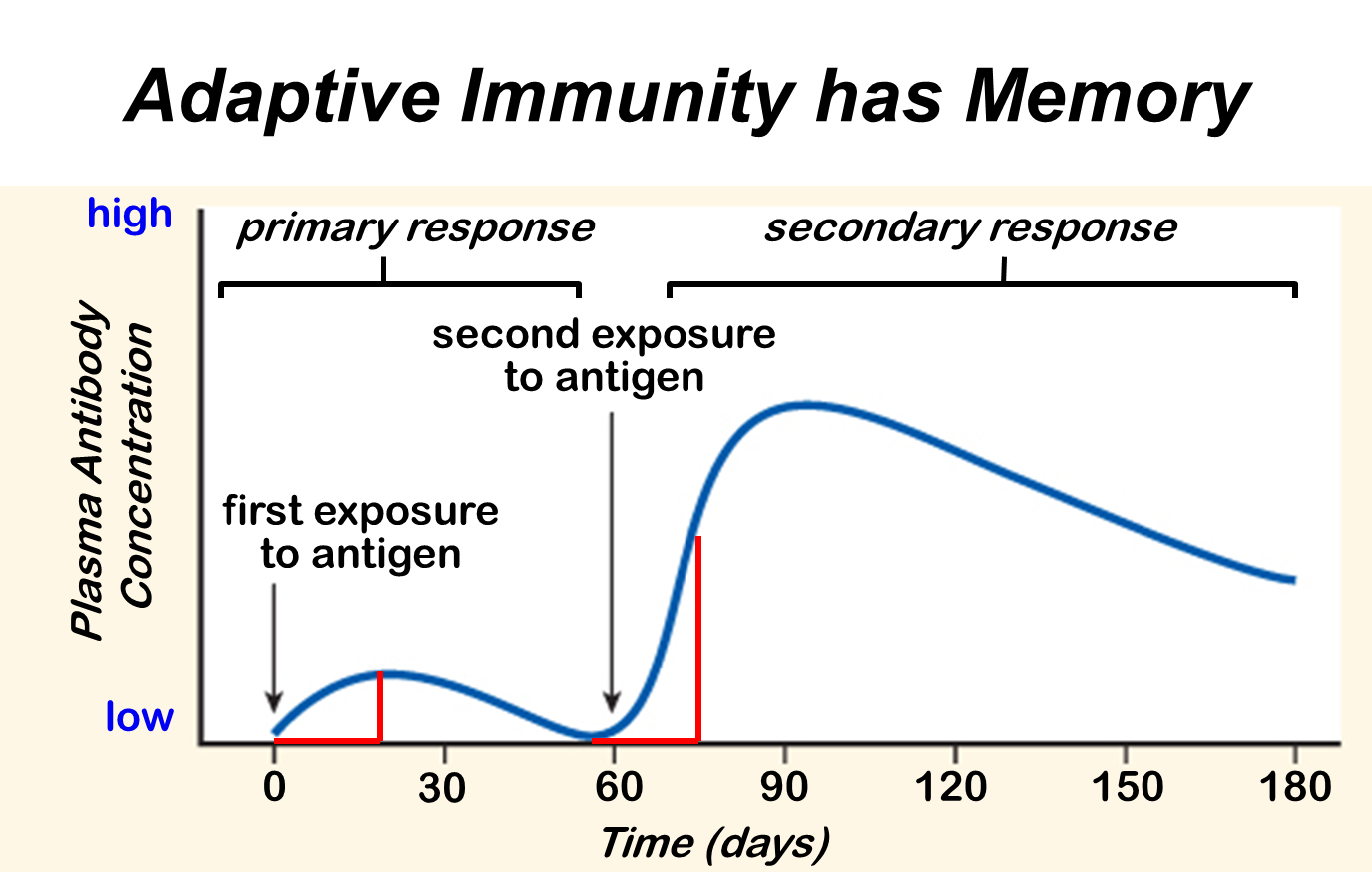
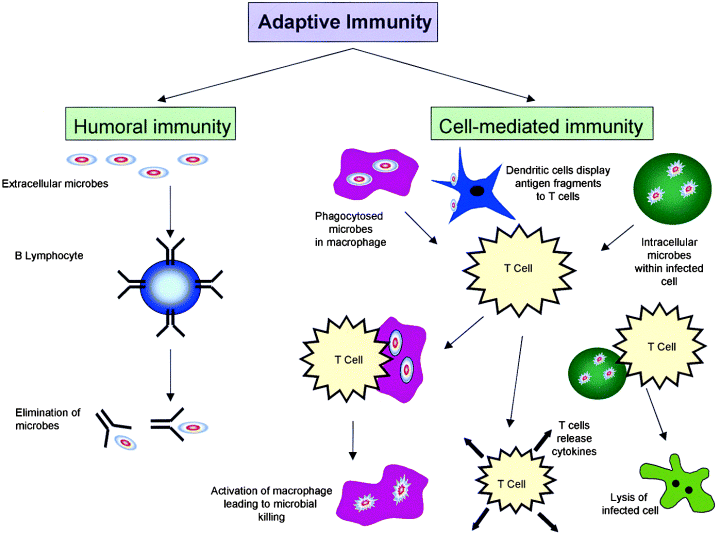







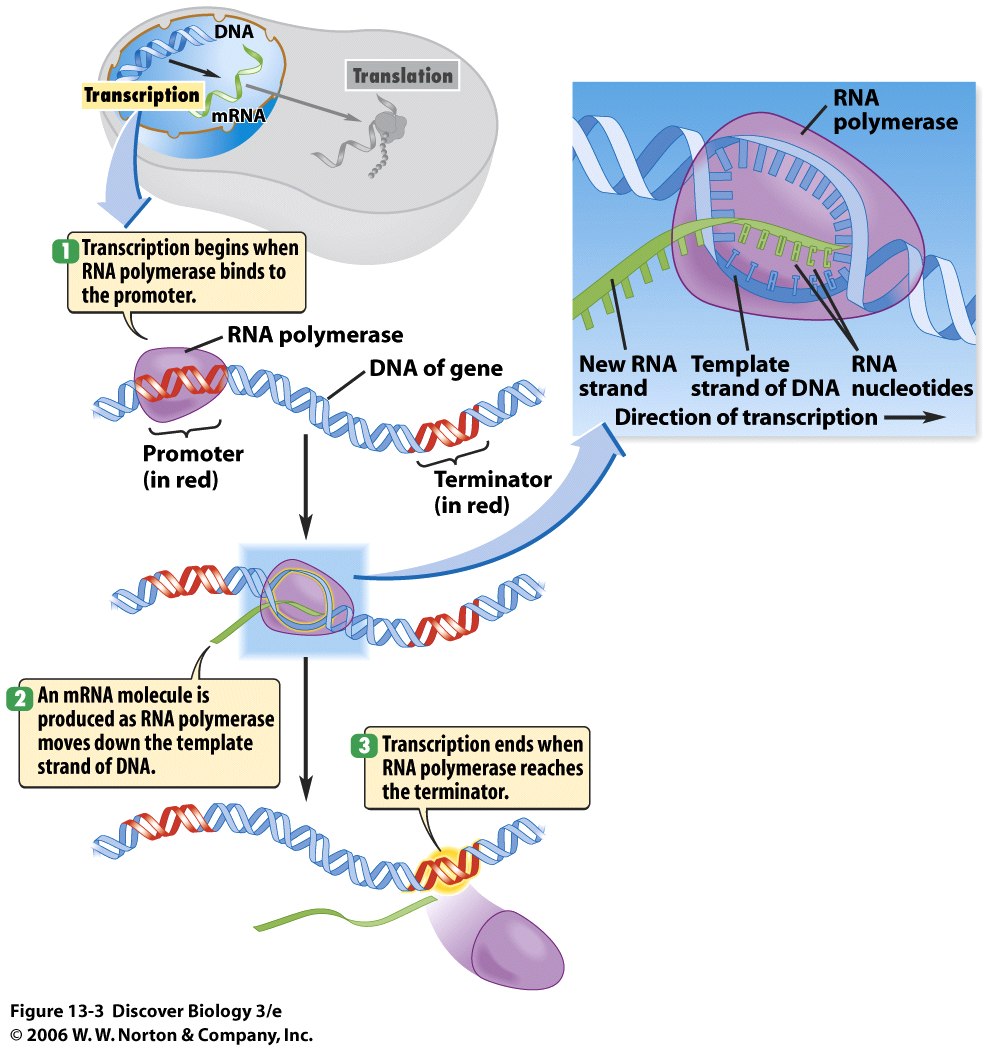


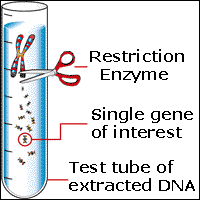
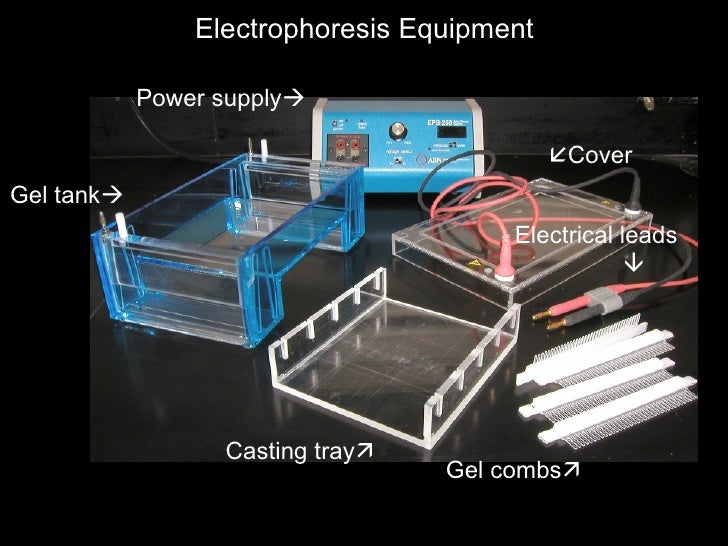





 Here is an example of a sucker.
Here is an example of a sucker.