VLP's Are the Key to Vaccinations
Imagine a world where immunogenicity is offered to every individual all at a price of a vaccination shot. Thousands to millions of lives could be saved from deadly, epidemic diseases just by taking a single shot. Is it worth it? This is the world that I want to pursue and hopefully with the help of Nicotiana benthamiana plants it will be possible. In this article that I have read, which is called Plant-derived Virus-like Particles as Vaccines, there are specialized particles called virus-like particles (VLP's) that are used to help the host immune system develop immunity against a certain surface protein or antigen. However, something to point out VLP's only help develop immunity in a host only against pathogenic viruses and not bacteria. These VLP's derived their unique structure from viral antigens that mimic the general structure of the real viruses, however these VLP's lack the viral genome. Due to a high presentation of viral antigens on a natural virus' surface, VLP's are able to copy these antigens and mimic the antigens on their surface. This result demonstrates that VLP's are valuable in vaccinations for humans, because our immune system just has to encounter these antigens for a first time, before our immune system develops antibodies that are best suited to those specific antigens' structure. The major problem to VLP's is the amount of production costs, but in this article a solution has been made.
The lab plants, Nicotiana benthamiana, are a cheaper alternative to producing these VLP's, because of the low production costs, low maintenance costs, and high scalability factors that are offered by these plants. The production of VLP's in plants is a quicker process than other mechanism because the transgenic plants that I am working with grow quickly when placed in the right laboratory conditions: high humidity, water tank, fertilizer, and a healthy soil amount. It takes around two weeks for the plants to grow to the max controlled height that we want the plants to be up to, so that shows to tell you that these plants grow really quickly. What is so remarkable about these plants is that according to a theory about vaccine transportation; plants that hold the VLP's can be ingested in edible plant parts such as the leaves in order to transmit the VLP's particles orally to a host. However, this is only a theory and has been only tested in lab mice. Also, to point out there are several concerns over this delivery system such as possible denaturation occurring to the proteins that make up the antibodies due to the low pH in the human digestion system, poor recognition of the vaccine at mucosal immune effector sites, and antigenic tolerance. As you can see the biggest hurdle to using plants as an antibody production site is the delivery method of the vaccination to a host. In the article, the author mentioned that the most reasonable delivery method would be direct injection using a syringe needle, which is the most common alternative for any vaccination. In the next paragraph, I will be discussing how the VLP's develop immunity for hosts like humans against the actual deadly pathogens.
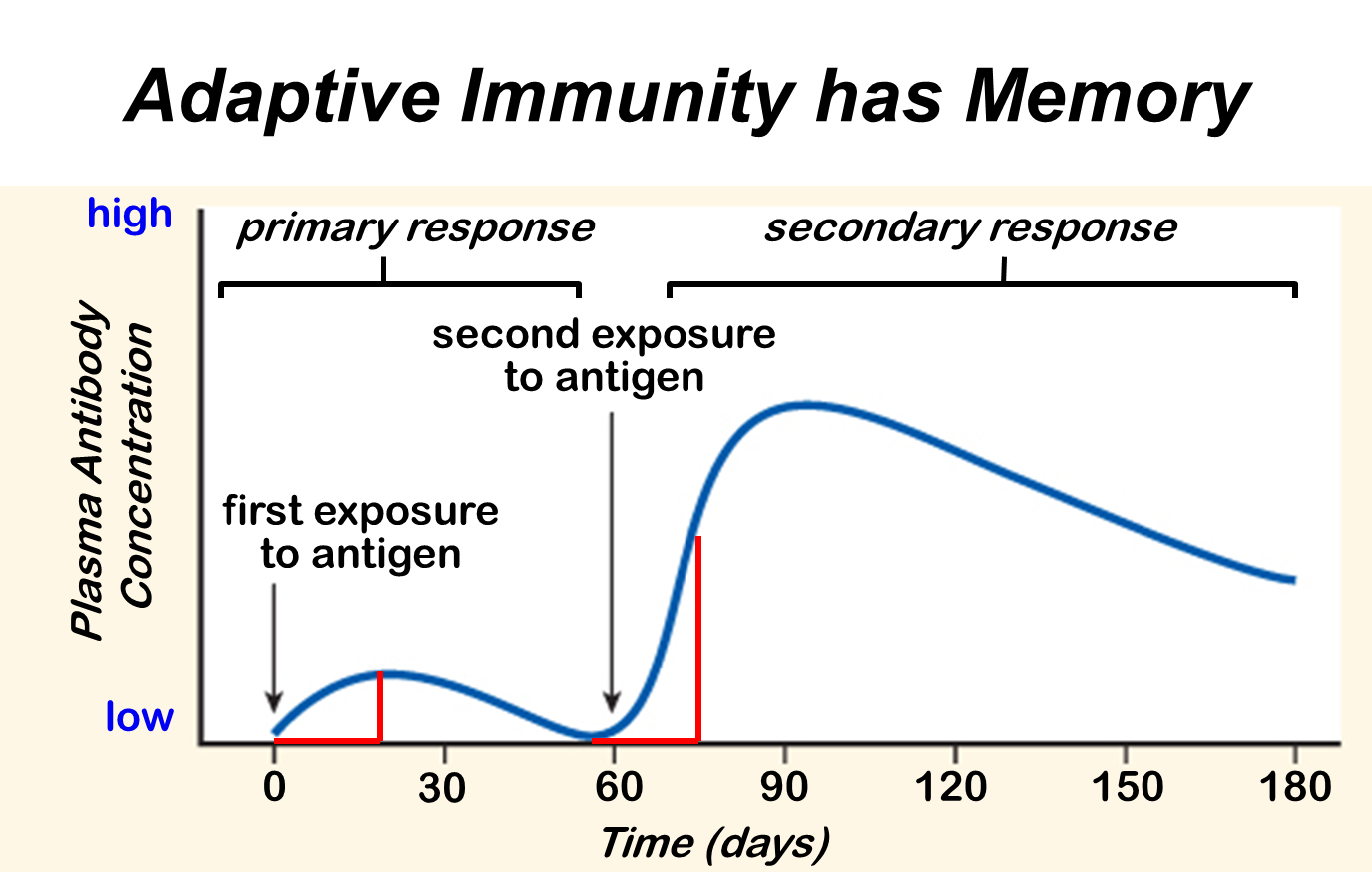
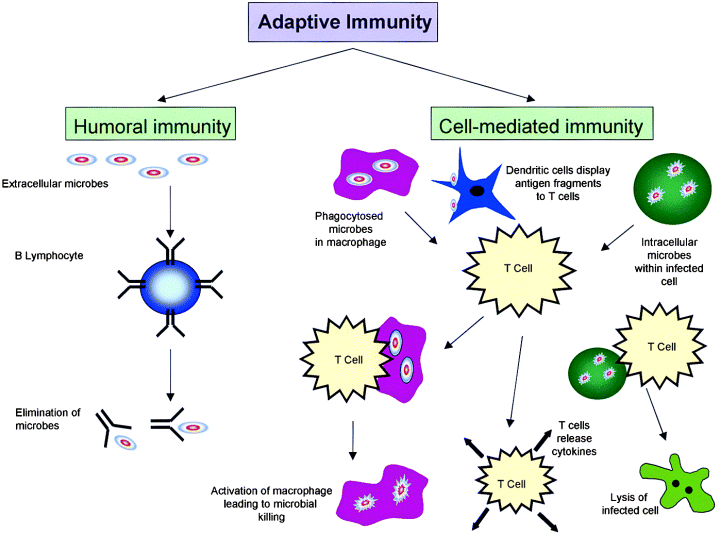
The way the VLP's are structured is the main reason why these simple particles can help hosts develop immunity against the wild type viruses. Since VLP's are particulate it allows them to induce T-cell mediated immune responses due to their interaction with the antigen presenting cells (APC) found in the host's immune system. Remember T cells are specialized immune cells that work to destroy a foreign cell by signaling the infected cells to undergo apoptosis, which means the cell self-destructs and dies. This action is vital to a host's defenses, because of how effective this process can be directed against pathogens. In order for the VLP's to induce the T cell activation they have to mimic the process of a natural infection, where the antigens presented by the VLP's should trigger a cascade immune event where the host's immune system attacks the VLP's. As the immune system destroys the VLP's, since the VLP's have no mechanism to infect the host's cells, the B cells should develop "memory" against the specific antigen structures that were presented by the VLP's. In turn, the host's immune system would develop immunity against the actual pathogenic virus, since the immune cells can quickly develop an immune response against the pathogen quicker. Another reason that allows the VLP's to develop immunity in a host is the presence of epitopes on the cell surface. Epitopes are a specific region on an antigen that is recognized by the host's immune system and to which where an antibody binds to. The presence of thousands of epitopes on a single VLP aids to the processing and presentation of APC's. What else is so interesting about these VLP's is that some of them are so small that they can diffuse to the lymph nodes, allowing the VLP's to be presented efficiently by B and T cells. That is all that is to it to VLP's and how they help develop immunity in hosts. These particles are very efficient at their job and hopefully become more available to be used in all future vaccinations.